Bipolar Disorder
Lithium: Cellular Trickster
Psychiatry's oldest drug still has secrets to be revealed.
Posted December 31, 2018
A while back, antioxidants were all the rage. Vitamin E could cure dementia, they said. Vitamin C prevents colds! The more the better! Big studies and a lot of research was done on these various vitamins, and for the most part it came to nothing. Other antioxidant studies have been disappointing as well. Part of the reason is that these vitamins aren’t actually antioxidants…we make our own antioxidants (like glutathione). It turns out that these vitamins may work by inspiring our cells toward recovery and repair by being a little bit…well…poisonous. This is a process called hormesis, where a little bit of a stress or chemical makes you stronger, but a lot of it can cause problems. Athletes will use brief ice baths to improve recovery, but stay in an ice bath for a little too long, and you turn into a popsicle.
Lithium is an essential mineral that appears to be extremely helpful to the brain in low doses, but is definitely toxic if you get the dose too high. The “essential mineral” dose is quite low (maybe 2-5mg daily), whereas a therapeutic dose of lithium for, say, bipolar disorder, will depend on how someone metabolizes it, and a specific blood level range is reached usually at elemental doses of approximately 100-270mg (roughly corresponding to 600-1500mg of a prescribed dose of lithium carbonate). Go much above this, and you get confusion, slowed heart rate, and kidney failure.
A recent interesting paper suggests that lithium may well act as those antioxidant vitamins, tricking the cells into activating neuron protection, recovery and repair. Along the way it increases cellular “second messenger” systems, beefing up communication in the cell rather like having extra riders during those long middle sections of a cross country Pony Express route back in the day. These second messengers are known as the inositol phosphates. How lithium does this has been a bit of a mystery…does it fool the cell into thinking it is sodium and prevents more intense ion gradients shooting through the cell membranes? This paper suggests an even more basic but very elegant mechanism…that lithium neatly tricks the brain into thinking that it is low in magnesium without causing the damage that low magnesium can.
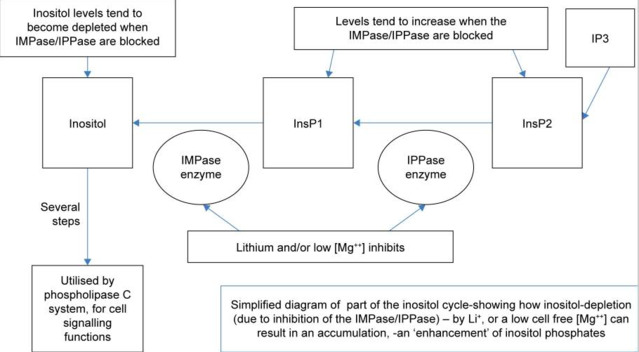
Magnesium is another extremely important mineral for heart, muscle, and brain. Magnesium is an antioxidant, keeps cells from dying after damage and also keeps cells from a type of cell suicide called apoptosis. Turns out the ion lithium is about the same size as the ion of magnesium floating around between cells. They are both positively charged (lithium has the same charge as sodium as well, though sodium is much bigger). Lithium can imitate magnesium without acting as magnesium, fooling the cell into thinking magnesium levels have dropped and causing the cell to initiate a fail-safe recovery and repair to protect it from the supposed drop in magnesium levels. Lithium can do this without actually dropping magnesium levels (which can be dangerous). A lot of magnesium dependent enzymes that help with recovery and repair can be activated by lithium too.
For example, in a traumatic brain injury, magnesium levels in the cells can abruptly drop to half normal or more, associated with tissue injury and lower levels of energy production in the cell. This is part of the reason neurons can die with a brain injury. Lithium, then, may be useful in traumatic brain injury treatment.
There is, however, a catch. The more some of these fail-safe enzymes are activated, the less the mechanism seems to work. The reason for this is, well, complicated and involves yet another positive mineral ion, calcium. It seems that the cell has to draw a line between preserving magnesium from further losses and allowing too much calcium into the cell (which can be toxic, especially in a neuron damage situation, as calcium and magnesium can compete as well…). The article is free full text if you want to dive into the details of the mechanisms.

Better understanding lithium mechanisms could help us establish appropriate treatments and doses for many conditions beyond bipolar disorder, which requires a high dose and careful monitoring to prevent toxicity. Lower, less dangerous doses, even the micro doses seen in certain populations with high levels in the water, are associated with less suicide, violence, and greater longevity.
Lithium continues to unwind brain mysteries in 2018 and beyond.
Special thank you to Dr. Woerkom who sent me his interesting paper via airmail.
Copyright Emily Deans MD


